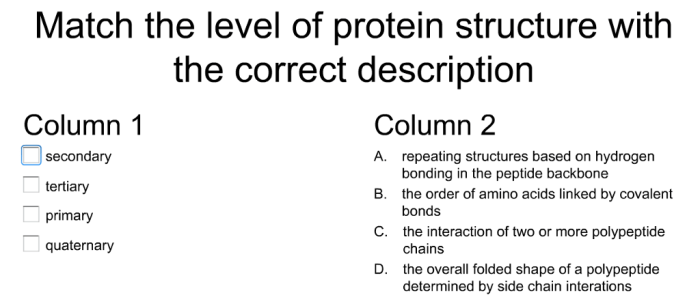
Match the level of protein organization with the proper description – Proteins, the workhorses of life, exhibit a remarkable hierarchy of structural organization, ranging from the simple arrangement of amino acids to the intricate assembly of multiple subunits. This article embarks on a journey to unravel the intricate relationship between the level of protein organization and its corresponding description, delving into the primary, secondary, tertiary, and quaternary structures that define the architecture of these biological marvels.
The primary structure, the foundation of protein architecture, comprises a linear chain of amino acids linked by peptide bonds. Secondary structures, such as alpha-helices and beta-sheets, emerge from the folding of the primary structure, stabilized by hydrogen bonding. Tertiary structures, more complex and compact, result from further folding and interactions, including hydrophobic interactions, disulfide bonds, and hydrogen bonding.
Finally, quaternary structures arise when multiple protein subunits assemble, held together by various interactions.
Protein Structure: Match The Level Of Protein Organization With The Proper Description
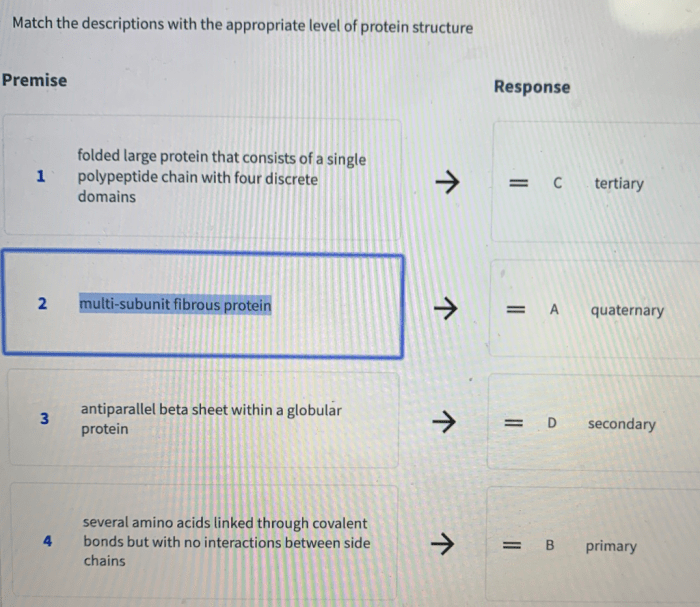
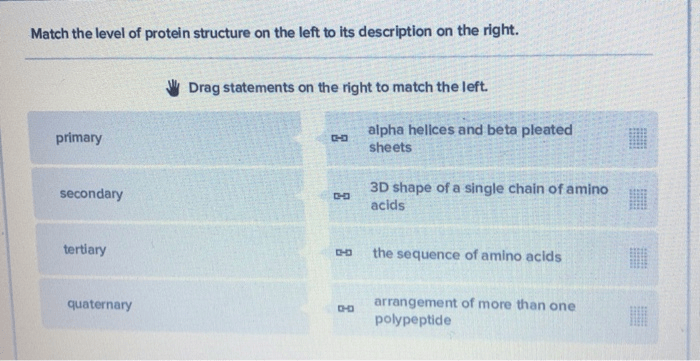
Proteins are essential biomolecules that perform a wide range of functions in living organisms. Their structure plays a crucial role in determining their function, and can be classified into four levels of organization: primary, secondary, tertiary, and quaternary.
Primary Structure
The primary structure of a protein refers to the linear sequence of amino acids that are linked together by peptide bonds. Each amino acid has an amino group, a carboxyl group, and a side chain with unique properties. The sequence of amino acids in the primary structure determines the protein’s unique identity and function.
Secondary Structure
The secondary structure of a protein refers to the regular, repeating patterns that form within the primary structure. The two main types of secondary structures are alpha-helices and beta-sheets. Alpha-helices are characterized by a helical shape stabilized by hydrogen bonds between the backbone NH and CO groups of every fourth amino acid.
Beta-sheets, on the other hand, are characterized by a pleated sheet-like structure stabilized by hydrogen bonds between the backbone NH and CO groups of adjacent strands.
Tertiary Structure
The tertiary structure of a protein refers to the three-dimensional arrangement of the secondary structure elements. This structure is determined by various forces, including hydrophobic interactions, disulfide bonds, and hydrogen bonding. Hydrophobic interactions drive the formation of a hydrophobic core within the protein, while disulfide bonds and hydrogen bonds stabilize the overall structure.
The tertiary structure is crucial for the protein’s function, as it determines the specific interactions that the protein can make with other molecules.
Quaternary Structure, Match the level of protein organization with the proper description
The quaternary structure of a protein refers to the arrangement of multiple protein subunits into a larger, functional complex. This level of organization is only found in proteins that are composed of multiple polypeptide chains. The subunits are held together by various interactions, such as hydrophobic interactions, hydrogen bonds, and electrostatic interactions.
The quaternary structure is essential for the function of many proteins, as it allows for cooperative interactions between the subunits.
Helpful Answers
What is the primary structure of a protein?
The primary structure is the linear sequence of amino acids linked by peptide bonds.
How does hydrogen bonding contribute to protein structure?
Hydrogen bonding stabilizes both secondary structures (alpha-helices and beta-sheets) and tertiary structures.
What forces contribute to tertiary structure formation?
Hydrophobic interactions, disulfide bonds, and hydrogen bonding are the primary forces responsible for tertiary structure formation.